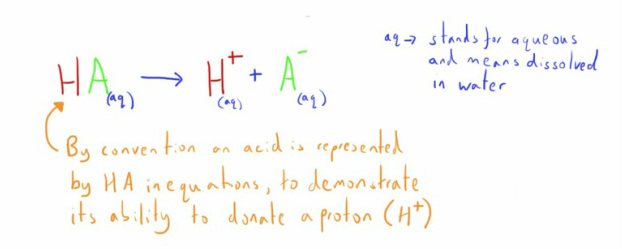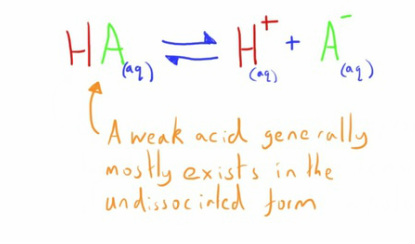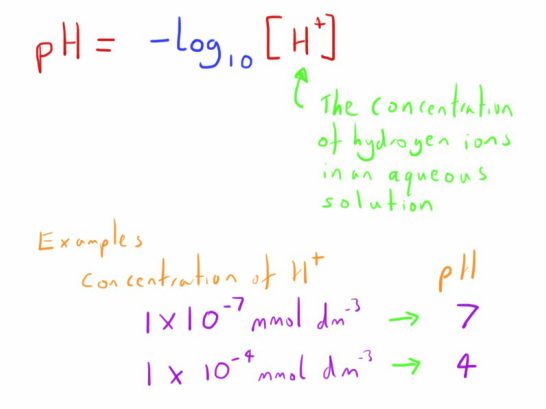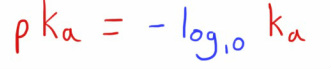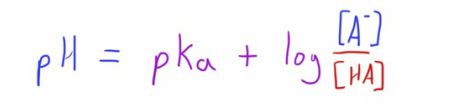Acids & Bases
Acids and bases play a very important role in physiology.
Through the Bronsted-Lowry definition (I think the most useful) we can understand that:
Through the Bronsted-Lowry definition (I think the most useful) we can understand that:
- an acid is a proton donor
- a base is a proton acceptor
The proton is commonly represented as H+; a hydrogen ion.
In reality it is actually a hydrogen ion attached to a water molecule forming a hydronium ion (H30+).
It is the concentration of H+ in a solution that represents the acidity we are interested in.
In reality it is actually a hydrogen ion attached to a water molecule forming a hydronium ion (H30+).
It is the concentration of H+ in a solution that represents the acidity we are interested in.
Strong Acids
A strong acid is where there is almost complete dissociation of the H+ from the acid.
Weak Acids
By comparison, weak acids only partially dissociate.
As such they can be considered to exist in an equilibrium.
In weak acids this equilibrium is generally far to the left i.e. little dissociation so little free H+ ions.
As such they can be considered to exist in an equilibrium.
In weak acids this equilibrium is generally far to the left i.e. little dissociation so little free H+ ions.
pH
The concentration of hydrogen ions in aqueous solution is what determines acidity.
In nature this concentration can vary dramatically (by a factor of over 1000000000000000!!)
The pH scale is therefore used to overcome the problems of having such a wide range.
The pH is simply the negative logarithm of the concentration of hydrogen ions.
In nature this concentration can vary dramatically (by a factor of over 1000000000000000!!)
The pH scale is therefore used to overcome the problems of having such a wide range.
The pH is simply the negative logarithm of the concentration of hydrogen ions.
A logarithm uses a base of 10 (though other bases exist) to convert the scale of the numbers used based on a factor of 10.
Ka and pKa
As we have discussed, weak acids form an equilibrium.
As such, if we can know about how much the weak acid dissociates, we can have an idea of much H+ they will create (which is what we are generally interested in).
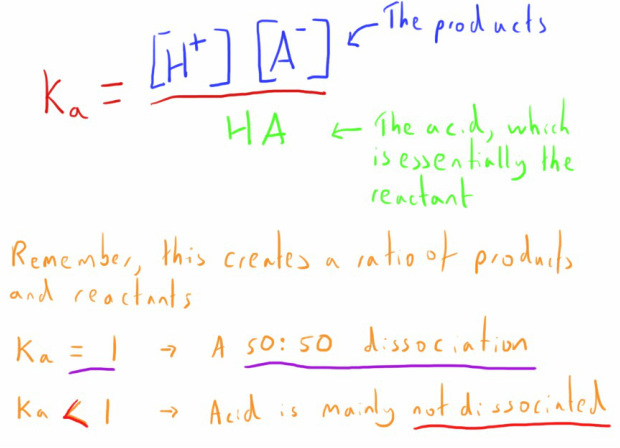
We can calculate the equilibrium constant of this reaction in the same way as we described we would calculate an equilibrium for another reaction (see the notes on equilibrium).
Instead of Kc, this is now denoted as Ka.
As such, if we can know about how much the weak acid dissociates, we can have an idea of much H+ they will create (which is what we are generally interested in).
We can calculate the equilibrium constant of this reaction in the same way as we described we would calculate an equilibrium for another reaction (see the notes on equilibrium).
Instead of Kc, this is now denoted as Ka.
This raises some important ideas:
As you can readily see, both of these will have an impact on determining the pH.
A weak acid in high concentrations can create a more acidic solution than a very low concentration of strong acid.
Weak acids generally have a Ka of less than 1
- The strength of the acid refers to how much it dissociates (i.e. how much H+ it donates)
- The concentration of the acid refers to how much of the molecule exists in the solution
As you can readily see, both of these will have an impact on determining the pH.
A weak acid in high concentrations can create a more acidic solution than a very low concentration of strong acid.
Weak acids generally have a Ka of less than 1
As with pH, it is often easier to change the scale of this number by using a negative logarithm conversion.
This is done by the same calculation, creating the pKa
This is done by the same calculation, creating the pKa
The Henderson-Hasslebach Equation
Why is all this information about acids and pKa important?
Well it is often relevant in pharmacology, as several important drugs are weak acids or weak bases.
Thus, an understanding of how they act in different environments can be grasped from this information about pKa.
Well it is often relevant in pharmacology, as several important drugs are weak acids or weak bases.
Thus, an understanding of how they act in different environments can be grasped from this information about pKa.
The Henderson-Hasslebach equation brings this together in a useful form.
The equation is shown below but if you want to see how it was put together (I think it helps with the understanding) click here.
The equation is shown below but if you want to see how it was put together (I think it helps with the understanding) click here.
How does this help us?
Well it tells us the position of the equilibrium of the acid at a particular pH, if we know the pKa of the particular weak acid.
When the pH is the same as the pKa, the ratio of the equilibrium is 50:50.
This can be seen by adding these values into the equation.
For the equation to work for this, the values of A- and HA have to be the same, i.e. the position of the equilibrium is 50:50 (this is because the log of 1 is 0).
Well it tells us the position of the equilibrium of the acid at a particular pH, if we know the pKa of the particular weak acid.
When the pH is the same as the pKa, the ratio of the equilibrium is 50:50.
This can be seen by adding these values into the equation.
For the equation to work for this, the values of A- and HA have to be the same, i.e. the position of the equilibrium is 50:50 (this is because the log of 1 is 0).
So now we know that when a drug is at a pH that is the same as its pKa, it will be dissociated to a ratio of 50:50.
Depending on the pH that is actually at, which may vary with exposure to acid in the stomach for instance, we can have an idea where the equilibrium of the reaction will lie.
This has important consequences on the drug, as it will behave differently in the ionised form (A-) when compared to the unionised form (HA).
Depending on the pH that is actually at, which may vary with exposure to acid in the stomach for instance, we can have an idea where the equilibrium of the reaction will lie.
This has important consequences on the drug, as it will behave differently in the ionised form (A-) when compared to the unionised form (HA).
An easy way to remember how weak acids and bases react with respect to pH is:
- Acids are more ionised at a pH Above their pKa
- Bases are more ionised at a pH Below their pKa