Atomic Structure
Atoms are composed of a variety of three sub-atomic particles:
The number of protons determines the element, and is denoted by the atomic number.
The number of protons and neutrons together determine the mass of the atom (each has a relative mass of 1), and hence is denoted by the mass number.
Different isotopes of an atom have different numbers of neutrons, and therefore different mass numbers.
Neutrons and protons are tightly packed together to form the nucleus of the atom.
- Positively charged protons
- Neutrally charged neutrons
- Negatively charged electrons
The number of protons determines the element, and is denoted by the atomic number.
The number of protons and neutrons together determine the mass of the atom (each has a relative mass of 1), and hence is denoted by the mass number.
Different isotopes of an atom have different numbers of neutrons, and therefore different mass numbers.
Neutrons and protons are tightly packed together to form the nucleus of the atom.
Electrons
Electrons have negative charge and negligible weight.
They orbit the nucleus in different shells that orbit at increasing distances from the nucleus.
Each shell contains a different number of electrons.
Shells are filled from the bottom up, and numbered in this way.
They orbit the nucleus in different shells that orbit at increasing distances from the nucleus.
Each shell contains a different number of electrons.
Shells are filled from the bottom up, and numbered in this way.
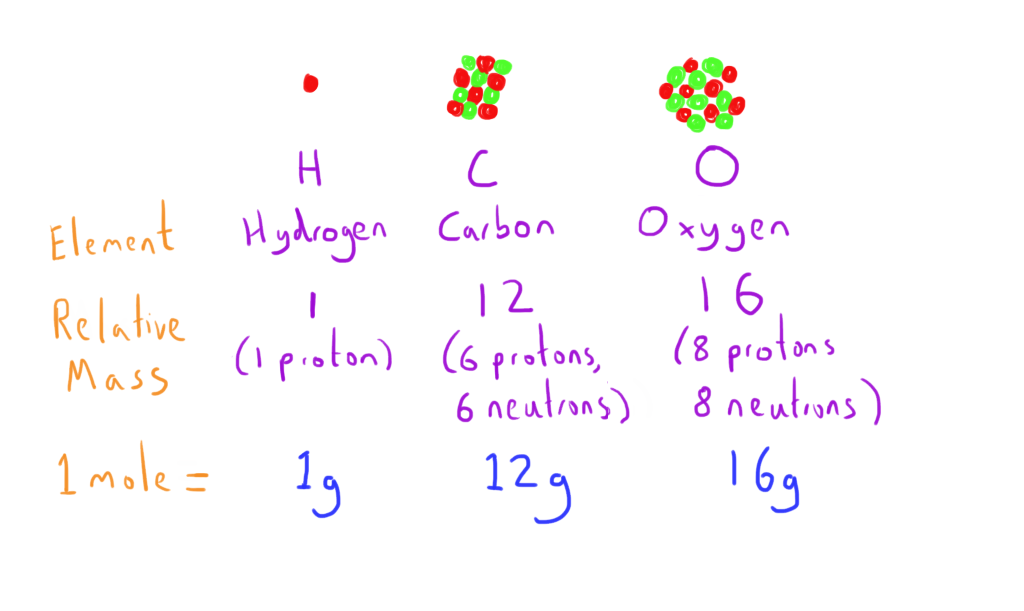
The Mole
The mole is a concept that allows an easy representation of the number of molecules present.
The mass of an atom is determined by the mass of the nucleus i.e. the mass number.
1 mole = the number of molecules in (mass number x 1) grams of an element
e.g. 1 mole of hydrogen (mass number = 1 ; 1 proton) is 1 gram
1 mole of carbon (mass number = 12 ; 6 protons, 6 neutrons) is 12 grams
1 mole is 6.02 x 10^23 molecules
That's about 602000000000000000000000 molecules!
The term mole can be applied to molecules as well as elements.
Here it will be the sum of the mass numbers that is relevant.
The mass of an atom is determined by the mass of the nucleus i.e. the mass number.
1 mole = the number of molecules in (mass number x 1) grams of an element
e.g. 1 mole of hydrogen (mass number = 1 ; 1 proton) is 1 gram
1 mole of carbon (mass number = 12 ; 6 protons, 6 neutrons) is 12 grams
1 mole is 6.02 x 10^23 molecules
That's about 602000000000000000000000 molecules!
The term mole can be applied to molecules as well as elements.
Here it will be the sum of the mass numbers that is relevant.