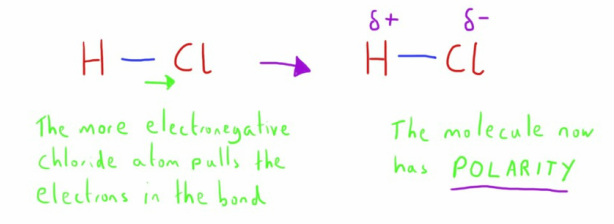Bonding
Atoms want to have the electron configuration of a noble gas (in most cases):
They want to have a full outer electron shell.
In basic terms, chemical bonds are based on this concept.
There are three types of chemical bonds:
They want to have a full outer electron shell.
In basic terms, chemical bonds are based on this concept.
There are three types of chemical bonds:
- Ionic
- Covalent
- Metallic
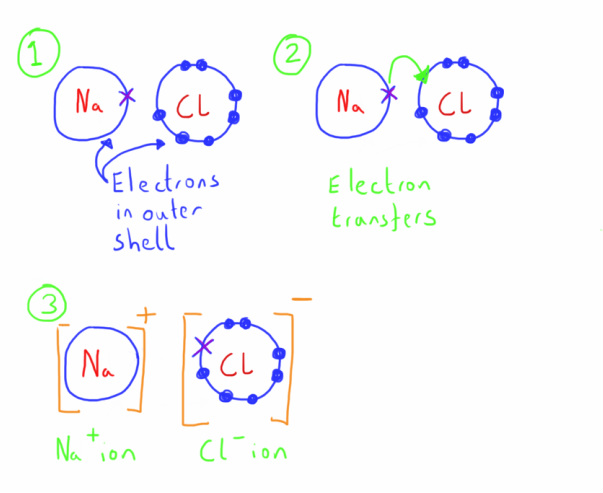
Ionic
These bonds form between a metal and a non metal.
One electron is transferred between the atoms (usually from metal to non-metal).
The atoms will both now have a full outer electron shell and will be ions (they have an electrical charge due to the imbalance that there now is between the number of protons and electrons of the atom)
The electrical attraction between these opposing ions constitutes the bond.
This electrical attraction occurs in all directions, thus forming large structure called giant ionic lattices.
One electron is transferred between the atoms (usually from metal to non-metal).
The atoms will both now have a full outer electron shell and will be ions (they have an electrical charge due to the imbalance that there now is between the number of protons and electrons of the atom)
The electrical attraction between these opposing ions constitutes the bond.
This electrical attraction occurs in all directions, thus forming large structure called giant ionic lattices.
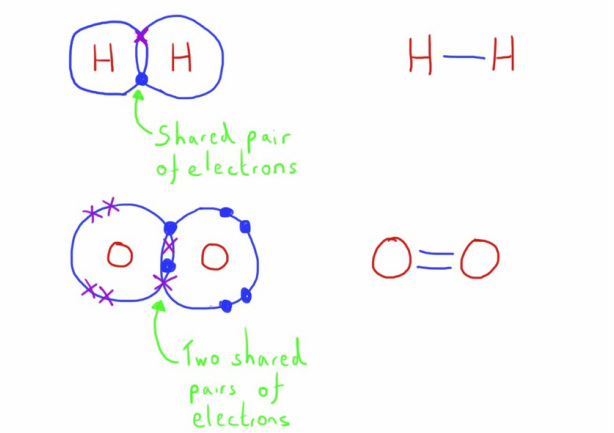
Covalent
These bonds form between non metal elements.
A covalent bond is formed when a pair of electrons is shared by both atoms.
Atoms therefore form molecules.
More than one pair of electrons can be shared in a bond; this can form double bonds or even triple bonds.
A covalent bond is formed when a pair of electrons is shared by both atoms.
Atoms therefore form molecules.
More than one pair of electrons can be shared in a bond; this can form double bonds or even triple bonds.
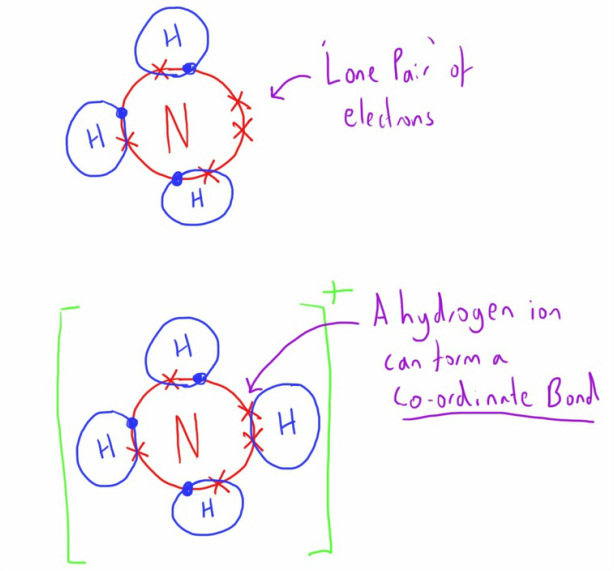
A particular type of covalent bond exists called a Co-ordinate bond.
It has the same structure as a covalent bond (i.e. a shared pair of electrons) but in this case both the electrons are donated by just one of the molecules.
Several important molecules in human physiology have atoms with a free pair of electrons in their outer shell that have the potential to form a co-ordinate bond with another atoms that will accommodate 2 electrons (a hydrogen ion being a good example).
It has the same structure as a covalent bond (i.e. a shared pair of electrons) but in this case both the electrons are donated by just one of the molecules.
Several important molecules in human physiology have atoms with a free pair of electrons in their outer shell that have the potential to form a co-ordinate bond with another atoms that will accommodate 2 electrons (a hydrogen ion being a good example).
In the example above, the nitrogen atom has a 'lone pair' of electrons.
As such it has been able to donate these electrons to a hydrogen ion (with an empty outer electron shell) to form a co-ordinate bond.
In this case it will form an ionic molecule (an ammonium ion) as it has a charge.
As such it has been able to donate these electrons to a hydrogen ion (with an empty outer electron shell) to form a co-ordinate bond.
In this case it will form an ionic molecule (an ammonium ion) as it has a charge.
Metallic
Less relevant to physiology.
Electrons dissociate from the nucleus.
The nuclei exist in a 'sea of electrons' which provide strong electrical attraction.
Electrons dissociate from the nucleus.
The nuclei exist in a 'sea of electrons' which provide strong electrical attraction.
Impure bonding
In a pure ionic bond, there is complete electron transfer between the atoms.
In a pure covalent bond, the pair of electrons is shared completely equally by the atoms.
As such, these can be considered to be two ends of a spectrum.
In reality, bonds often exist somewhere along this spectrum rather than having a pure ionic or covalent characteristic.
In a pure covalent bond, the pair of electrons is shared completely equally by the atoms.
As such, these can be considered to be two ends of a spectrum.
In reality, bonds often exist somewhere along this spectrum rather than having a pure ionic or covalent characteristic.
Electronegativity determines the degree of ionic properties of a covalent bond.
Electronegativity is a measure for the strength of attraction that an atom has for the electrons in the covalent bond.
In general, the smaller an atom is, the greater it's electronegativity.
This is due to there being less distance between the attractive positive charge of the protons in the nucleus, and less electrons shielding the nucleus with their negative charge.
Electronegativity is a measure for the strength of attraction that an atom has for the electrons in the covalent bond.
In general, the smaller an atom is, the greater it's electronegativity.
This is due to there being less distance between the attractive positive charge of the protons in the nucleus, and less electrons shielding the nucleus with their negative charge.
Polar bonds
Polarity occurs in covalent bonds when there is a difference in the electronegativity of the atoms involved in the bond.
The atom with the greater electronegativity will exert a greater pull on the electrons in the bond, as can be best seen in covalent bonds.
This gives this atom a slightly more negative charge, compared with the other atom which will now have a slight positive charge.
The molecule will therefore be a polar molecule with a permanent dipole.
The atom with the greater electronegativity will exert a greater pull on the electrons in the bond, as can be best seen in covalent bonds.
This gives this atom a slightly more negative charge, compared with the other atom which will now have a slight positive charge.
The molecule will therefore be a polar molecule with a permanent dipole.
Some good examples of this:
- In a hydrogen chloride molecule the chlorine is more electronegative than hydrogen, making it a polar molecule with the chlorine atom having a slight negative charge.
- In a hydrogen molecule the two atoms (obviously) have the same electronegativity, and so the molecule is non polar.