Calcium Physiology
This is a nice video of the physiology:
https://www.youtube.com/watch?v=EEM0iRJNhU8
The introduction of the video from the Osmosis team on hypocalcaemia is also reallyhelpful: https://www.youtube.com/watch?v=KWZrSYo7xuk
https://www.youtube.com/watch?v=EEM0iRJNhU8
The introduction of the video from the Osmosis team on hypocalcaemia is also reallyhelpful: https://www.youtube.com/watch?v=KWZrSYo7xuk
Calcium is an important extracellular cation in the body.
The vast majority (99%) of calcium and phosphate in the body is stored within the bone as hydroxyapatite (Ca10[PO4]6[OH]2).
Only a small percentage circulates in the plasma, but it is this amount which has many important physiological effects, and which is regulated by the body’s homeostatic mechanisms.
This interchange between the bone stores and plasma is regulated by the action of osteoBlasts (B for building bone) and osteoClasts (C for clearing bone).
A fraction of the total body calcium (0.01%) is intracellular.
Calcium in the plasma exists in several forms.
About 45% exists as the ‘free’, and physiologically active, ionised form.
About another 40% circulates bound to albumin. This calcium is termed non-diffusible.
The final 15% is complexed with moieties such as citrate, phosphate, oxalate or sulphate, which although ‘diffusible’ are not active.
The total calcium that is measured on blood testing includes all of these forms, and may be subject to error in cases where there is low albumin.
These forms are clearly in relationship to each other.
The calcium levels can be changed depending on the albumin levels, and also the ability of the albumin to bind calcium.
With a rise in pH, the hydrogen atoms off the albumin’s carboxyl groups move towards the equilibrium of disassociation.
This leaves more of the negatively charged COO- sites to bind to calcium, thus lowering the levels of the ionised calcium.
The vast majority (99%) of calcium and phosphate in the body is stored within the bone as hydroxyapatite (Ca10[PO4]6[OH]2).
Only a small percentage circulates in the plasma, but it is this amount which has many important physiological effects, and which is regulated by the body’s homeostatic mechanisms.
This interchange between the bone stores and plasma is regulated by the action of osteoBlasts (B for building bone) and osteoClasts (C for clearing bone).
A fraction of the total body calcium (0.01%) is intracellular.
Calcium in the plasma exists in several forms.
About 45% exists as the ‘free’, and physiologically active, ionised form.
About another 40% circulates bound to albumin. This calcium is termed non-diffusible.
The final 15% is complexed with moieties such as citrate, phosphate, oxalate or sulphate, which although ‘diffusible’ are not active.
The total calcium that is measured on blood testing includes all of these forms, and may be subject to error in cases where there is low albumin.
These forms are clearly in relationship to each other.
The calcium levels can be changed depending on the albumin levels, and also the ability of the albumin to bind calcium.
With a rise in pH, the hydrogen atoms off the albumin’s carboxyl groups move towards the equilibrium of disassociation.
This leaves more of the negatively charged COO- sites to bind to calcium, thus lowering the levels of the ionised calcium.
The normal range for total plasma calcium is 2.25-2.65 mmol/L.
It has a number of essential physiological functions, including:
As noted, intracellular calcium levels are usually kept very low.
Indeed, calcium entry into cells is part of the apoptosis pathway.
This low level is maintained by active transport of calcium out of the cells, through calcium pumps and sodium-calcium exchanger pumps.
The intracellular calcium that is present is primarily stored within the different organelles e.g. endoplasmic reticulum.
This allows some control of the calcium levels for specific tasks e.g. release for muscular contraction.
Phosphorus is found in the plasma in both inorganic and organic forms.
The inorganic phosphate is found in the plasma completely ionised as either HPO4-- (80%) or H2PO4- (20%).
It has a number of essential physiological functions, including:
- Secondary messenger intracellularly
- Muscle contraction
- Haemostasis
- Membrane excitation
- Hormone secretion
- Structural support (cellularly as well as in bone)
As noted, intracellular calcium levels are usually kept very low.
Indeed, calcium entry into cells is part of the apoptosis pathway.
This low level is maintained by active transport of calcium out of the cells, through calcium pumps and sodium-calcium exchanger pumps.
The intracellular calcium that is present is primarily stored within the different organelles e.g. endoplasmic reticulum.
This allows some control of the calcium levels for specific tasks e.g. release for muscular contraction.
Phosphorus is found in the plasma in both inorganic and organic forms.
The inorganic phosphate is found in the plasma completely ionised as either HPO4-- (80%) or H2PO4- (20%).
Absorption
Body calcium is absorbed from dietary sources via the GI tract.
This occurs via two mechanisms:
Partly this is because of the combination with other moieties in the gut to form insoluble salts, but the absorption mechanism is also vitamin D dependent.
The TRPV6 (transient receptor potential vanilloid 6) is the channel found in the duodenum and proximal jejunum where the active transcellular movement occurs.
This occurs via two mechanisms:
- Paracellular transport through the length of the gut
- An active transcellular pathway
Partly this is because of the combination with other moieties in the gut to form insoluble salts, but the absorption mechanism is also vitamin D dependent.
The TRPV6 (transient receptor potential vanilloid 6) is the channel found in the duodenum and proximal jejunum where the active transcellular movement occurs.
Excretion
As noted, as certain amount is simply lost from the gut through a failure to absorb - about 18 mmol of the total of 20 mmol ingested.
This amount can be regulated to some degree through the action of PTH.
The kidneys remain the main site of regulated excretion.
Much of the filtered ionised calcium is reabsorbed passively in the PCT or loop of Henle.
However, about 10-15% is under active reabsorption control in the more distal part of the nephron (DCT through the TRVPV5 channel) and can be adjusted to regulate calcium levels.
This amount can be regulated to some degree through the action of PTH.
The kidneys remain the main site of regulated excretion.
Much of the filtered ionised calcium is reabsorbed passively in the PCT or loop of Henle.
However, about 10-15% is under active reabsorption control in the more distal part of the nephron (DCT through the TRVPV5 channel) and can be adjusted to regulate calcium levels.
Homeostasis
There are 3 key hormones involved:
These have their actions through a number of tissues:
- Parathyroid hormone (PTH)
- Vitamin D
- Calcitonin (less important)
These have their actions through a number of tissues:
- Parathyroid gland
- Thyroid gland
- Kidneys
- Liver
- Skin
- GI tract
- Bone
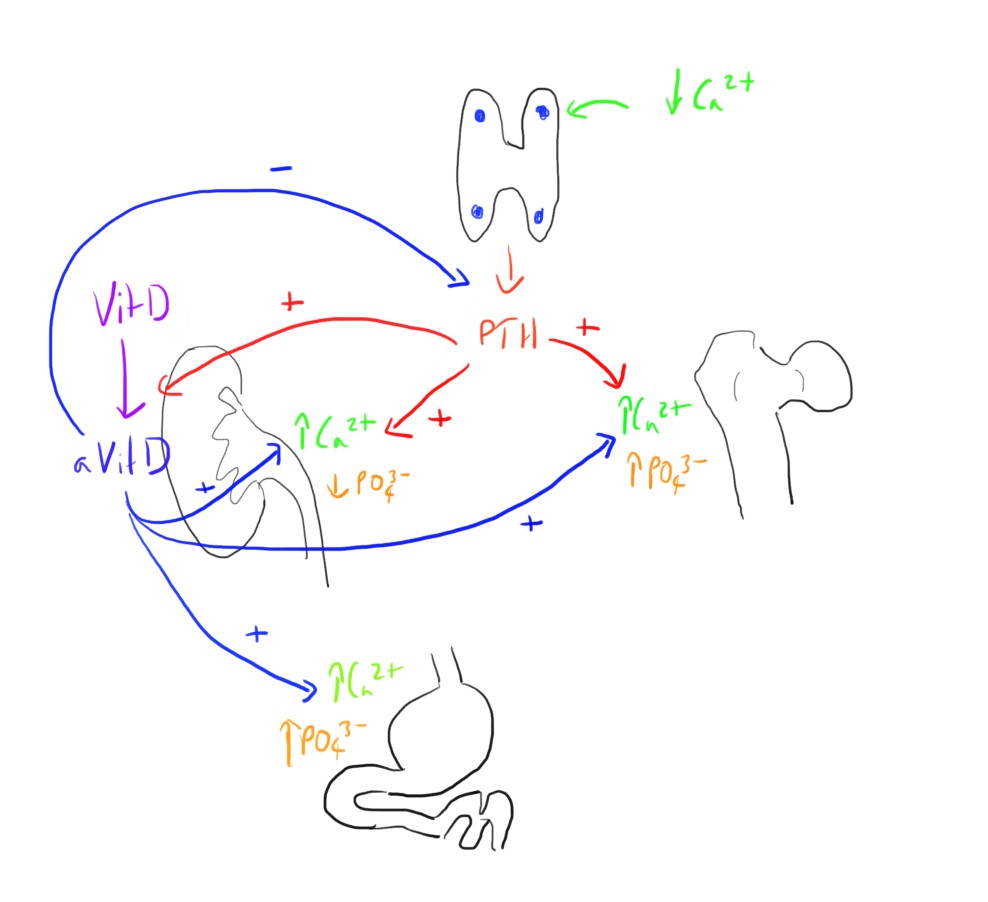
Parathyroid Hormone
This is produced by the chief cells of the parathyroid glands in response to a drop in plasma calcium levels, sensed be the calcium sensing receptor on the cells.
This has a number of direct and indirect actions on calcium and phosphate, raising plasma calcium levels and lowering phosphate levels.
It results in:
This has a number of direct and indirect actions on calcium and phosphate, raising plasma calcium levels and lowering phosphate levels.
It results in:
- Release of calcium and phosphate from bone
- Increased renal reabsorption of calcium
- Decreased renal reabsorption of phosphate
- Activation of vitamin D
Vitamin D
This can be seen as another very important part of the homeostasis of calcium, but there are a few different molecules in the pathway to be aware of.
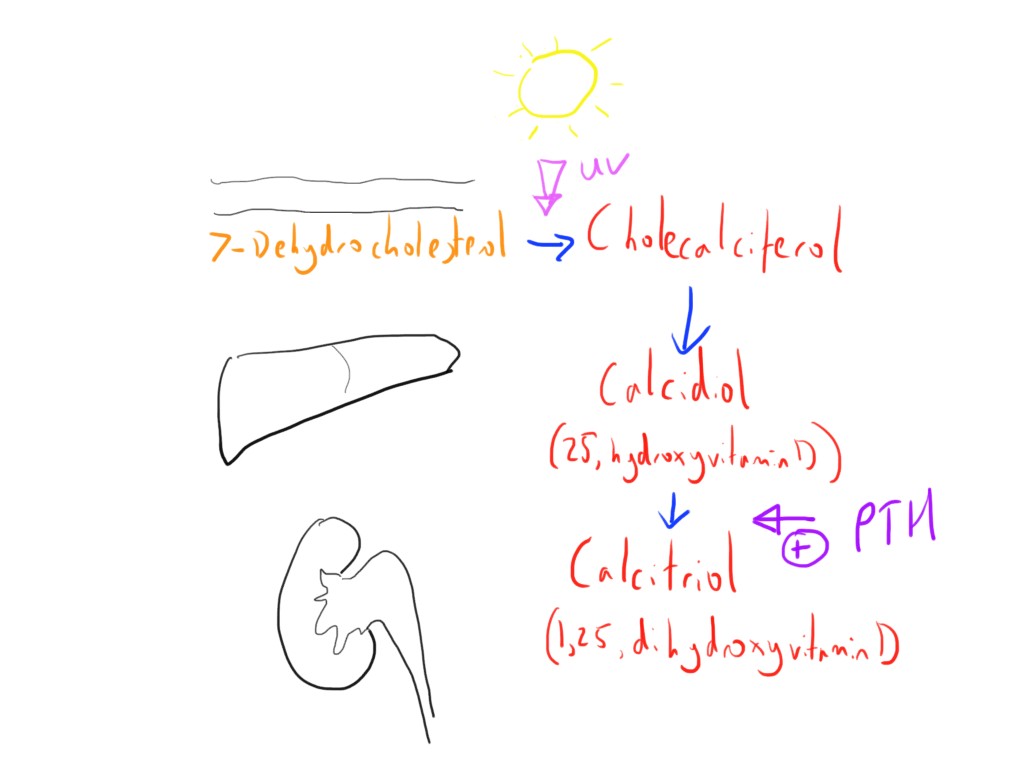
Vitamin D3 (cholecalciferol) is initially synthesised in the skin by the action of UV light from the sun on 7-dehydrocholesterol.
It is fairly common that there is inadequate sunlight for this synthesis, and so may also need to be ingested orally.
The next step of metabolism occurs in the liver, where it is hydroxylated by the enzyme 25-hydroxylase to form 25-hydroxyvitamin D (the 25 because the hydroxylation occurs at the 25th position) which is also called calcidiol. This is the most common form of vitamin D in the circulation.
The final step occurs in the kidney, where it is initially filtered at the glomerulus, reabsorbed at the PCT and then ‘activated’, stimulated by PTH, to form calcitriol (1,25-dihydroxyvitamin D).
There is also a pathway in the kidney where the vitamin D is inactivated (24,25-dihydroxyvitamin D).
The calcitriol is the form that has the most activity.
The actions include:
An additional source of calcitriol may arise from activated macrophages and thymic derived lymphocytes, which may play a role in the pathophysiology of some conditions e.g. sarcoidosis.
Vitamin D3 (cholecalciferol) is initially synthesised in the skin by the action of UV light from the sun on 7-dehydrocholesterol.
It is fairly common that there is inadequate sunlight for this synthesis, and so may also need to be ingested orally.
The next step of metabolism occurs in the liver, where it is hydroxylated by the enzyme 25-hydroxylase to form 25-hydroxyvitamin D (the 25 because the hydroxylation occurs at the 25th position) which is also called calcidiol. This is the most common form of vitamin D in the circulation.
The final step occurs in the kidney, where it is initially filtered at the glomerulus, reabsorbed at the PCT and then ‘activated’, stimulated by PTH, to form calcitriol (1,25-dihydroxyvitamin D).
There is also a pathway in the kidney where the vitamin D is inactivated (24,25-dihydroxyvitamin D).
The calcitriol is the form that has the most activity.
The actions include:
- Releasing calcium and phosphate from bone
- Increasing GI absorption of calcium and phosphate
- Increasing renal reabsorption of calcium
- Inhibiting PTH release (as part of a negative feedback loop)
An additional source of calcitriol may arise from activated macrophages and thymic derived lymphocytes, which may play a role in the pathophysiology of some conditions e.g. sarcoidosis.
Calcitonin
This is released from the parafollicular C-cells of the thyroid gland in response to a rise in plasma calcium levels.
The effects are essentially the opposite of PTH.
It partly acts by reducing osteoclast activity leading to less bone breakdown.
These effects are not fully understood, and it seems that much of the homeostatic response to high calcium is through a reduction in the activity of the other mechanism (PTH and Vitamin D).
The effects are essentially the opposite of PTH.
It partly acts by reducing osteoclast activity leading to less bone breakdown.
These effects are not fully understood, and it seems that much of the homeostatic response to high calcium is through a reduction in the activity of the other mechanism (PTH and Vitamin D).
Links & References
- Hasudungan, A. Endocrinology - calcium and phosphate regulation. Youtube. 2015. https://www.youtube.com/watch?v=EEM0iRJNhU8
- Hogan, J. Goldfarb, S. Regulation of calcium and phosphate balance. UpToDate. 2018.
- McIndoe, A. Thyroid and parathyroid hormones and calcium homeostasis. e-LFH. 2014.
- Power I, Kam P. Principles of Physiology for the Anaesthetist (2nd ed). 2008. Hodder Arnold.
- Osmosis. Hypocalcemia - causes, symptoms, diagnosis, treatment, pathology. Youtube. 2017. https://www.youtube.com/watch?v=KWZrSYo7xuk
- Tidy, C. Hypocalcaemia. Patient.info. 2015. https://patient.info/doctor/hypocalcaemia
- Nickson, C. Hypocalcaemia. LITFL. 2013. https://lifeinthefastlane.com/ccc/hypocalcaemia/