Diffusion
Last updated 26th February 2019 - Tom Heaton
After air enters the alveoli, the next step is to reach the blood.
This occurs solely by the process of diffusion.
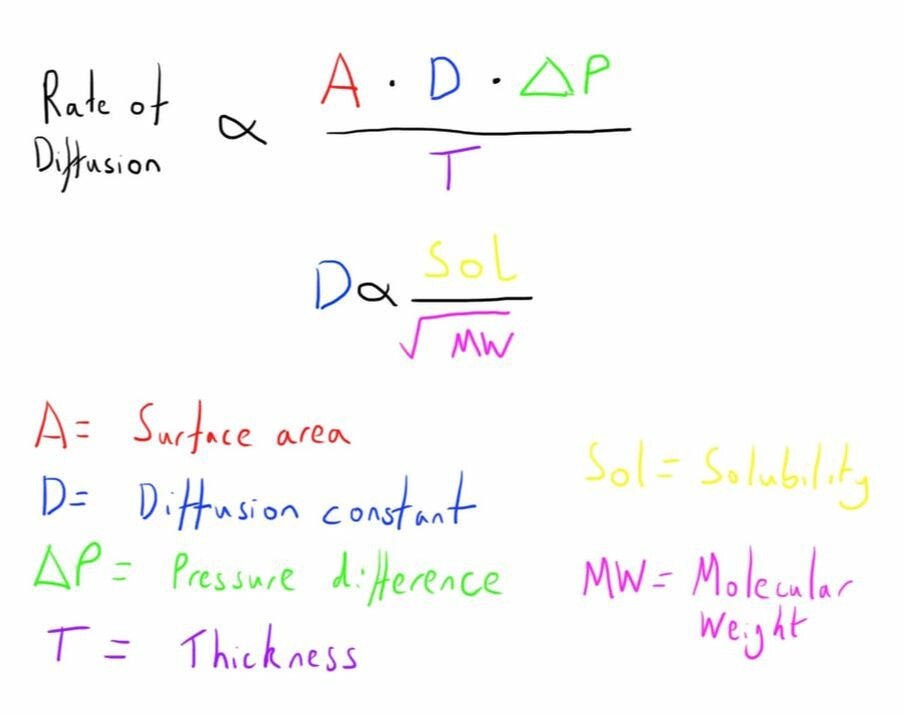
The key component of understanding this step is Fick’s law of diffusion.
This occurs solely by the process of diffusion.
The key component of understanding this step is Fick’s law of diffusion.
We can use this to understand some key concepts.
Molecular Differences
The component D in Fick’s law is the molecular specific component.
This is one factor as to how different gases may behave differently.
The key factor is the solubility of the gas in the membrane, whilst the molecular weight is also important, but has slightly less of an impact.
Of note to respiratory physiology, is the difference between CO2 and O2.
CO2 is much more soluble than O2, but there is minimal difference between their molecular weights.
As such, CO2 diffuses across the membrane 20 times faster than O2.
This means that different physiological and pathological factors will affect the levels of the two gases.
This is one factor as to how different gases may behave differently.
The key factor is the solubility of the gas in the membrane, whilst the molecular weight is also important, but has slightly less of an impact.
Of note to respiratory physiology, is the difference between CO2 and O2.
CO2 is much more soluble than O2, but there is minimal difference between their molecular weights.
As such, CO2 diffuses across the membrane 20 times faster than O2.
This means that different physiological and pathological factors will affect the levels of the two gases.
Limitations of Diffusion
As can be seen, a partial pressure gradient of the gas is required to ‘drive’ diffusion.
Some factors that impact on this gradient will therefore have an important role in the rates of diffusion.
An example is what happens to the gases when they reach the blood, as many of them act very differently.
A discussion of the two extremes allows demonstration of this.
Carbon monoxide (CO) is avidly bound to haemoglobin when it reaches the blood.
This means that it is very rapidly ‘taken out’ of the solution, and the partial pressure of the gas barely rises.
As such, the gas can continue to be ‘absorbed’ by the blood nearly indefinitely, and the the limiting factors to how much will diffuse will actually be the other components of Fick’s law.
As such, CO is a good example of being diffusion limited - the ability to diffuse across the membrane is what will limit the volume of gas transferred.
In contrast, nitrous oxide (NO2) is very insoluble when it gets to the blood side.
As a result, the partial pressure of the gas rises very rapidly, quickly stopping further diffusion.
In order for further diffusion to occur, the NO2 in the blood must be taken away, and the blood replaced by fresh blood with minimal or no NO2 in it.
As such, NO2 is a good example of being perfusion limited - the replacement of the blood in the lung unit is the limited factor in the volume of gas transferred.
Oxygen lies between these two extremes.
It binds rapidly with haemoglobin, and so the partial pressure drops to some degree.
However, the haemoglobin is rapidly saturated, and so the limitation is usually dependent on how quickly the haemoglobin can be replaced i.e. it is perfusion limited.
However, this reaction time is not as fast as, for example, with CO, and takes about 0.2s.
In normal condition, this in not significant, as the blood takes 0.75 seconds to pass through the lung capillaries.
In exercise though, blood flow is notably increased, and the speed of blood flow might not allow time for this reaction to occur (especially if there is disease of the lungs).
In these scenarios, the volume of oxygen that is transferred will actually be diffusion limited.
Some factors that impact on this gradient will therefore have an important role in the rates of diffusion.
An example is what happens to the gases when they reach the blood, as many of them act very differently.
A discussion of the two extremes allows demonstration of this.
Carbon monoxide (CO) is avidly bound to haemoglobin when it reaches the blood.
This means that it is very rapidly ‘taken out’ of the solution, and the partial pressure of the gas barely rises.
As such, the gas can continue to be ‘absorbed’ by the blood nearly indefinitely, and the the limiting factors to how much will diffuse will actually be the other components of Fick’s law.
As such, CO is a good example of being diffusion limited - the ability to diffuse across the membrane is what will limit the volume of gas transferred.
In contrast, nitrous oxide (NO2) is very insoluble when it gets to the blood side.
As a result, the partial pressure of the gas rises very rapidly, quickly stopping further diffusion.
In order for further diffusion to occur, the NO2 in the blood must be taken away, and the blood replaced by fresh blood with minimal or no NO2 in it.
As such, NO2 is a good example of being perfusion limited - the replacement of the blood in the lung unit is the limited factor in the volume of gas transferred.
Oxygen lies between these two extremes.
It binds rapidly with haemoglobin, and so the partial pressure drops to some degree.
However, the haemoglobin is rapidly saturated, and so the limitation is usually dependent on how quickly the haemoglobin can be replaced i.e. it is perfusion limited.
However, this reaction time is not as fast as, for example, with CO, and takes about 0.2s.
In normal condition, this in not significant, as the blood takes 0.75 seconds to pass through the lung capillaries.
In exercise though, blood flow is notably increased, and the speed of blood flow might not allow time for this reaction to occur (especially if there is disease of the lungs).
In these scenarios, the volume of oxygen that is transferred will actually be diffusion limited.
Links & References
- West. B. Respiratory physiology: the essentials (9th ed). 2012
- Cross, M. Plunkett, E. Physics, pharmacology and and physiology for anaesthetists: Key concepts for the FRCA. Cambridge University Press. 2010.