Equilibrium
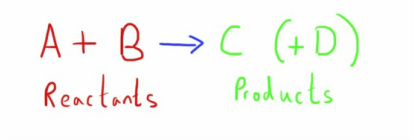
A simple equation in chemistry can be expressed as two or more reactants forming a product (or products).
However, not all reactions go to completion.
In many important circumstances, the reaction is also reversible, and the products can react to form the reactants.
In these cases, an equilibrium will exist.
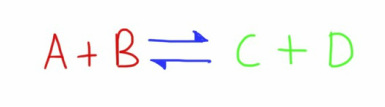
In many important circumstances, the reaction is also reversible, and the products can react to form the reactants.
In these cases, an equilibrium will exist.
In a closed system (when there can be no lost or gain of the reactants and products), a dynamic equilibrium can form.
This is when the rate of both the forward and backward reactions become the same.
At this point the amounts of both the products and reactants will remain the same, though in reality there is ongoing formation of products from the reactants and vice versa.
This is when the rate of both the forward and backward reactions become the same.
At this point the amounts of both the products and reactants will remain the same, though in reality there is ongoing formation of products from the reactants and vice versa.
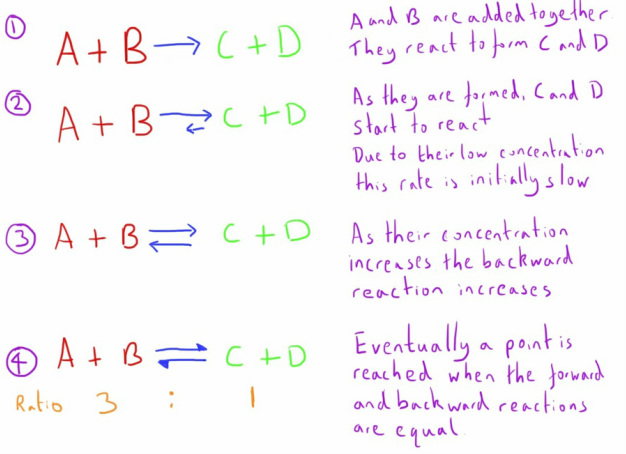
The position of the equilibrium, refers to the ratio of the amounts of reactants and products.
This will rarely be a 50:50 ratio, as one direction is likely to predominate.
This will rarely be a 50:50 ratio, as one direction is likely to predominate.
In the example, the ratio is 3:1
This is because, the backwards reaction (products to reactants) happens more easily that the forward reaction (reactant to products).
However, a dynamic equilibrium has developed because, as there are more molecules on the reactant side, collisions between them happen more frequently.
This means that although the reaction is less likely to happen when a collision occurs, the greater probability of collisions means that the total number of forward reactions will match the number of backward reactions.
This is because, the backwards reaction (products to reactants) happens more easily that the forward reaction (reactant to products).
However, a dynamic equilibrium has developed because, as there are more molecules on the reactant side, collisions between them happen more frequently.
This means that although the reaction is less likely to happen when a collision occurs, the greater probability of collisions means that the total number of forward reactions will match the number of backward reactions.
Le Chatelier's Principle
Simply put, if a system in dynamic equilibrium is subject to change the equilibrium will move to oppose this change.
This gives several results when changes are applied to a dynamic equilibrium:
- If the concentration of on of the molecules is increase (or decreased) then the equilibrium will move to the opposite side of the equilibrium.
- If the temperature of the environment is changed, the equilibrium will move in the direction that opposes the temperature changes. This is because one reaction will be exothermic (heat producing) whilst the reverse reaction will be endothermic (heat absorbing) to the same degree. For example, if the temperature is increased, the equilibrium will favour the reaction direction that is endothermic, as this opposes the change by taking in heat.
- If the pressure of a gas is increased, the equilibrium will move towards the side with the fewest molecules. Similarly, if the pressure is reduced, the equilibrium will move towards the side of the equilibrium with the greatest number of molecules.
The Equilibrium Constant
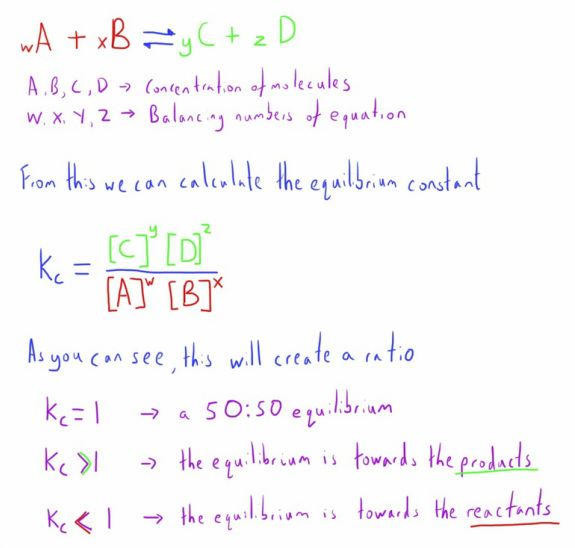
The position of the equilibrium can be calculated from the concentrations of the different components when they are measured at the point of equilibrium.
As it is a simple ratio it is calculated by dividing the products by the reactants.
This is traditionally referred to as the equilibrium constant, denoted by Kc.
The equation below demonstrates how this is calculated.
As it is a simple ratio it is calculated by dividing the products by the reactants.
This is traditionally referred to as the equilibrium constant, denoted by Kc.
The equation below demonstrates how this is calculated.
As such Kc can give information about where the equilibrium of a reaction exists, which is useful in certain medical scenarios such as weak acids, which we shall cover shortly.