Haemoglobin
Synthesis
Haemoglobin synthesis occurs in the developing red blood cell during the proerythroblast stage and continues into the reticulocyte stage when the blood cell first leaves the bone marrow and enters the blood stream.
Production does not occur in the mature red blood cell though.
Production does not occur in the mature red blood cell though.
We can follow the chain of events in the construction of haemoglobin to better understand its final structure and function.
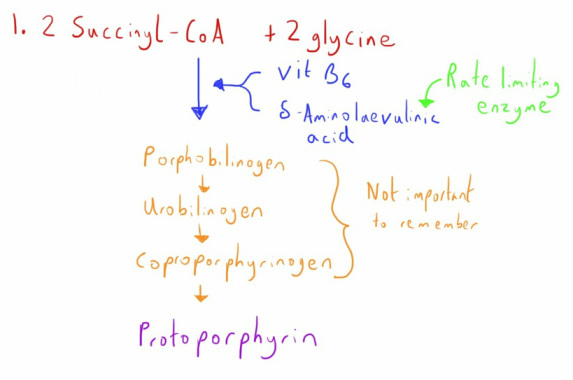
Firstly we create the protoporphyrin molecule which is the outer shell that holds the iron.
Firstly we create the protoporphyrin molecule which is the outer shell that holds the iron.
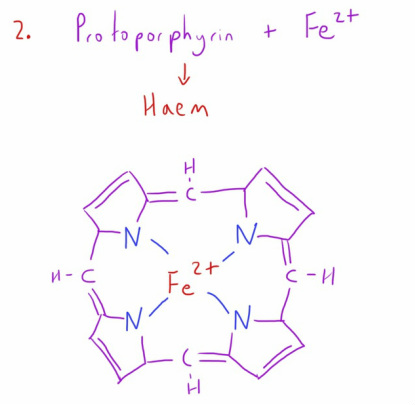
This is combined with iron in its ferrous state (2+) to create a haem molecule.
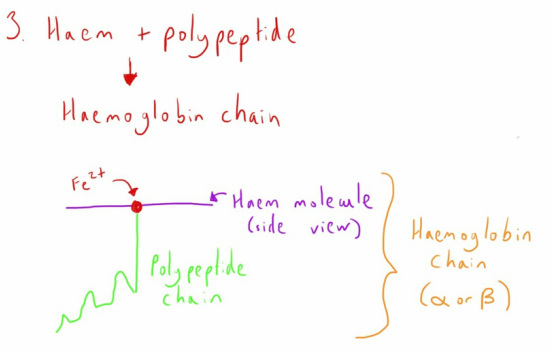
A polypeptide is then added to this molecule to form a haemoglobin chain. The nature of the polypeptide will determine what type of haemoglobin chain it is (e.g. an alpha or beta chain).
If you picture the haem molecule (as shown above) as a flat disc then the polypeptide chain attaches to the iron atom perpendicular to the plane of the molecule.
If you picture the haem molecule (as shown above) as a flat disc then the polypeptide chain attaches to the iron atom perpendicular to the plane of the molecule.
We now have the building blocks of haemoglobin.
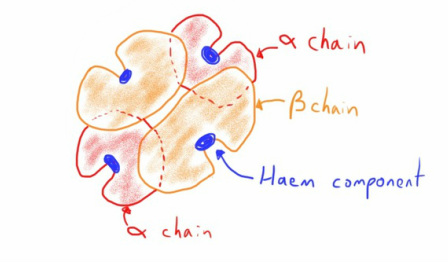
In adult haemoglobin (mostly haemoglobin A) it is made up of 2 alpha haemoglobin chains with 2 beta haemoglobin chains.
Therefore, final haemoglobin molecule is a quaternary protein with 4 subunits.
As you can see the polypeptide chains are slightly larger than the chain demonstrated in the diagram above.
In adult haemoglobin (mostly haemoglobin A) it is made up of 2 alpha haemoglobin chains with 2 beta haemoglobin chains.
Therefore, final haemoglobin molecule is a quaternary protein with 4 subunits.
As you can see the polypeptide chains are slightly larger than the chain demonstrated in the diagram above.
Oxygen Carriage
Hopefully it's also clear that each haemoglobin molecule has 4 haem components; one in each haemoglobin chain.
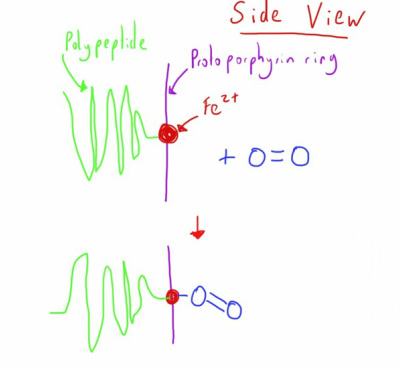
Each haem component can bind one molecule of oxygen, with the molecule binding to the Fe++ atom in the haem molecule, as below.
Each haemoglobin molecule can therefore carry 4 molecules of oxygen.
Each haem component can bind one molecule of oxygen, with the molecule binding to the Fe++ atom in the haem molecule, as below.
Each haemoglobin molecule can therefore carry 4 molecules of oxygen.