Intermolecular Forces
Intermolecular forces have a hugely important role in determining the physical properties of the molecules e.g. their melting point.
Covalent and ionic bonds are of comparable strength.
In comparison, intermolecular forces are weak.
There are several important types of intermolecular forces to understand:
Covalent and ionic bonds are of comparable strength.
In comparison, intermolecular forces are weak.
There are several important types of intermolecular forces to understand:
- Van Der Waals' forces
- Dipole-dipole interactions
- Hydrogen bonding
Van Der Waals' Forces
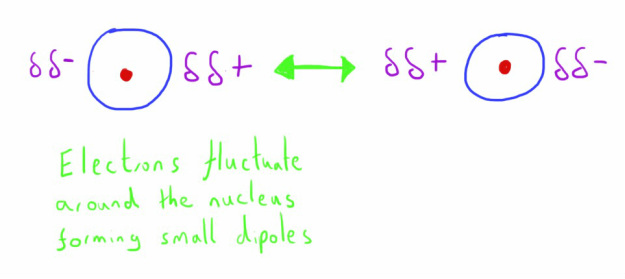
These are weak intermolecular forces that exist between all molecules.
They are caused by the attractions that exist between small, temporary induced dipoles that form in molecules.
They are caused by the attractions that exist between small, temporary induced dipoles that form in molecules.
These temporary dipoles form due to the nature of electrons orbiting around the nucleus in an uneven way.
At a particular moment (an incredibly small fraction of time), electron distribution around the nucleus will be greater on one side of the atom.
This gives that side of the atom a very small negative charge, with the opposite side a very slight positive charge.
The electrons are constantly moving around in this way, producing an oscillating dipole.
This oscillating dipole induces a dipole in neighbouring atoms.
These dipoles attract one another, providing a weak degree of intermolecular attraction.
At a particular moment (an incredibly small fraction of time), electron distribution around the nucleus will be greater on one side of the atom.
This gives that side of the atom a very small negative charge, with the opposite side a very slight positive charge.
The electrons are constantly moving around in this way, producing an oscillating dipole.
This oscillating dipole induces a dipole in neighbouring atoms.
These dipoles attract one another, providing a weak degree of intermolecular attraction.
As van der Waals' forces are the result of electron fluctuation, the greater the number of electrons, the stronger the van der Waals' forces.
This is because with a greater number of electrons the greater the oscillation that occurs and the larger the dipoles that result.
This is because with a greater number of electrons the greater the oscillation that occurs and the larger the dipoles that result.
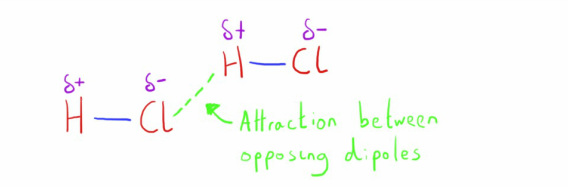
Dipole-dipole interactions
As described in the bonding chapter, some molecules have polar characteristics due to unequal distribution of the electrons in the covalent bond.
These small charges attract the opposing charges on neighbouring molecules.
These small charges attract the opposing charges on neighbouring molecules.
These molecules will also have van der Waals' between the molecules, but dipole-dipole forces are stronger (though still not comparable to ionic or covalent bonds).
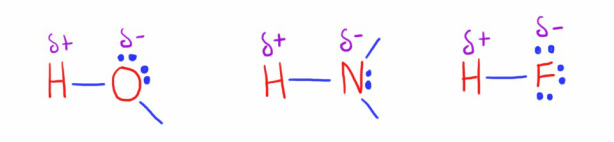
Hydrogen bonds
Hydrogen bonds are an important types of permanent dipole interactions, and comparatively strong for an intermolecular force.
They arise in molecules that have the following polar groups:
They arise in molecules that have the following polar groups:
- Hydrogen - oxygen
- Hydrogen - nitrogen
- Hydrogen - fluorine
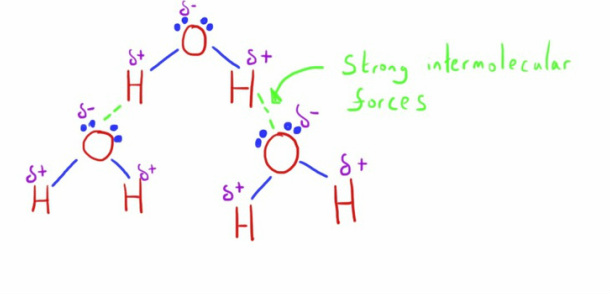
Hydrogen bonds a form by the attraction between the slight positive charge of the hydrogen atom to the lone pair of electrons on the highly electronegative atom of Oxygen, Nitrogen or Fluorine.
Hydrogen bonding in water is responsible for much of water's unique characteristics, due to strong intermolecular attraction between the hydrogen and oxygen atoms of different molecules.