Isomerism
Perhaps more relevant to pharmacology than physiology but still a very important part of organic chemistry that it is worth having some understanding of.
Isomerism refers to molecules which have the same chemical formula as each other but different chemical structures.
Basically they have the same building blocks but are put together in different ways.
There are two broad types of isomerism:
Basically they have the same building blocks but are put together in different ways.
There are two broad types of isomerism:
- Structural isomerism
- Stereoisomerism
Structural Isomerism
In this form of isomerism the order of the atoms and the bonding between them is different from each other.
This can range from small change to the carbon skeleton to the molecule having a completely different functional group and further sub-categorisation may help demonstrate this:
This can range from small change to the carbon skeleton to the molecule having a completely different functional group and further sub-categorisation may help demonstrate this:
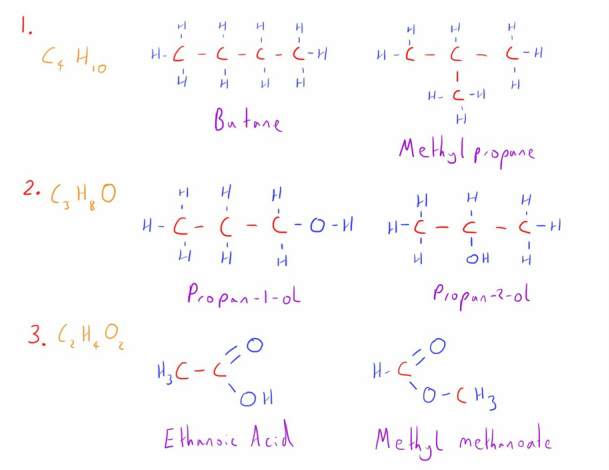
- Chain Isomerism - The carbon chain is different between the molecules
- Positional Isomerism - The functional group is simply in a different position on the carbon chain
- Functional Group Isomerism - A completely different functional group exists on each molecule
Tautomerism is a specific form of structural isomerism exhibited by a molecule.
The same molecule can interchange between two different chemical structures.
This is most commonly due to migration of a hydrogen atom and the shifting of a double bond position as a result.
The same molecule can interchange between two different chemical structures.
This is most commonly due to migration of a hydrogen atom and the shifting of a double bond position as a result.
Stereoisomerism
This form of isomerism refers to molecules that have the same bond structure as each other but the atoms have a different 3-dimentional configuration.
Two types exist:
Two types exist:
- Optical Isomerism (enantiomers)
- Diastereoisomers
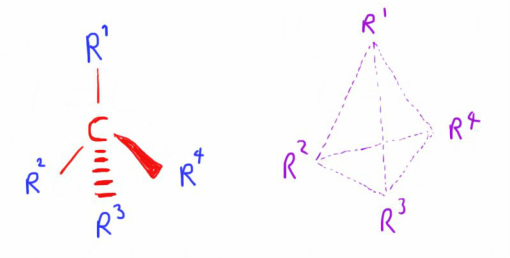
Optical Isomerism requires the presence of one or more Chiral Centres.
A chiral centre is an atom (usually carbon) that has 4 different groups bonded to it.
In 3-dimensions each of these bonds will exist as far away from each other as possible, thus the groups point to the vertices of a tetrahedron.
A chiral centre is an atom (usually carbon) that has 4 different groups bonded to it.
In 3-dimensions each of these bonds will exist as far away from each other as possible, thus the groups point to the vertices of a tetrahedron.
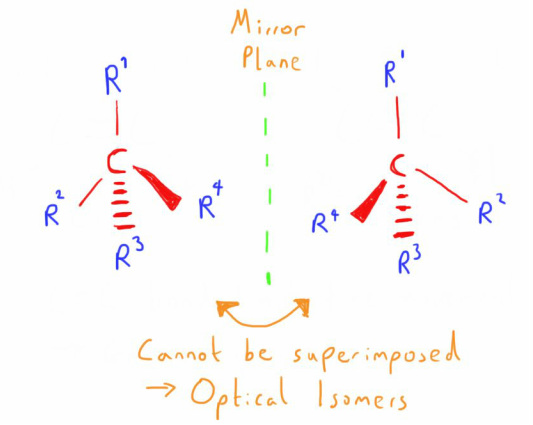
This structure allows 2 mirror images to exist.
These molecules cannot be imposed on each other and so are optical isomers.
This can be visualised most easily if you picture you left hand and your right hand as an example (the word chiral comes from the greek for hand).
They have exactly the same bits (fingers, thumb etc) and are a mirror image of each other, but they cannot be superimposed on each other.
These molecules cannot be imposed on each other and so are optical isomers.
This can be visualised most easily if you picture you left hand and your right hand as an example (the word chiral comes from the greek for hand).
They have exactly the same bits (fingers, thumb etc) and are a mirror image of each other, but they cannot be superimposed on each other.
The terminology to describe optical isomers is enantiomers.
They exist in an R (Rectus) or S (Sinister) form, referring to the mirror image molecules.
Drugs that are different enantiomers can exhibit notably different activity.
They exist in an R (Rectus) or S (Sinister) form, referring to the mirror image molecules.
Drugs that are different enantiomers can exhibit notably different activity.
Diastereomers are stereoisomers that are not mirror images of each other.
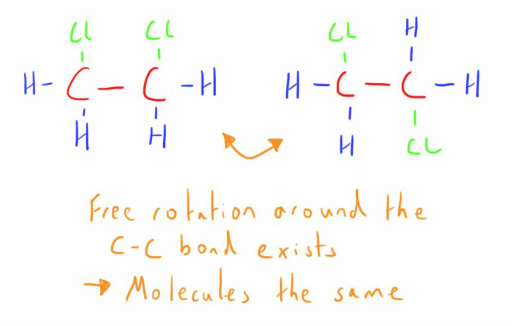
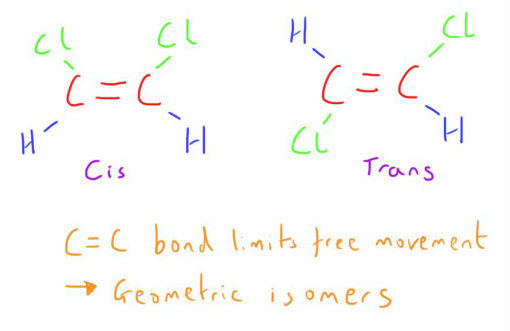
Geometric Isomers are one form of diasteromers and arise from the fact that, when an atom is connected to another atom (most commonly carbon) by a single bond, it is able to rotate freely around it.
When a double bond exists between 2 carbon atoms, or the carbon atoms are in a ring structure, atoms attached to these carbon atoms no longer have free movement.
If the groups or atoms attached to each carbon are dissimilar, then geometric isomers can exist.
If the groups or atoms attached to each carbon are dissimilar, then geometric isomers can exist.
If the groups are on the same side it is described as a cis-isomer, whilst if they are on the opposite side they are termed a trans-isomer.