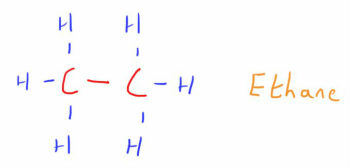Nomenclature
What better place to start than a simple run through of the nomenclature that is used through organic chemistry. I've found this to really help with understanding the descriptions of the different molecules that are encountered regularly throughout medical physiology and especially pharmacology.
Hydrocarbons
Hydrocarbons can be considered as the most basic components of the molecules encountered in organic chemistry.
They consist of molecules that contain only hydrogen and carbon atoms (hence their name).
They consist of molecules that contain only hydrogen and carbon atoms (hence their name).
The simplest organic compounds are the Alkanes.
These consist of a carbon skeleton, with each carbon atom bonded to each other by single bonds.
This carbon skeleton is then surrounded by hydrogen atoms at the free binding sites (each carbon atom can create 4 bonds)
These consist of a carbon skeleton, with each carbon atom bonded to each other by single bonds.
This carbon skeleton is then surrounded by hydrogen atoms at the free binding sites (each carbon atom can create 4 bonds)
The varying length of the carbon chain is denoted by a specific prefix, as described below.
As these are all alkanes, the suffix of the compound is '_ane'.
As these are all alkanes, the suffix of the compound is '_ane'.
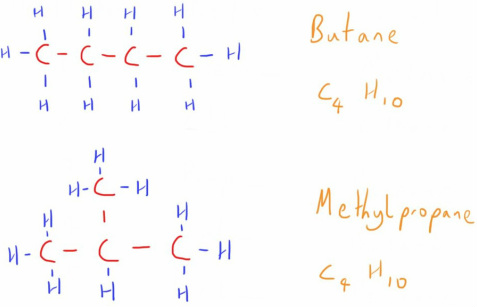
They don't have to simply form long chains though, and are good examples of structural isomerism (check out the page on isomerism for more about the different types).
This is where molecules have the same chemical formula but different structural arrangement.
This is where molecules have the same chemical formula but different structural arrangement.
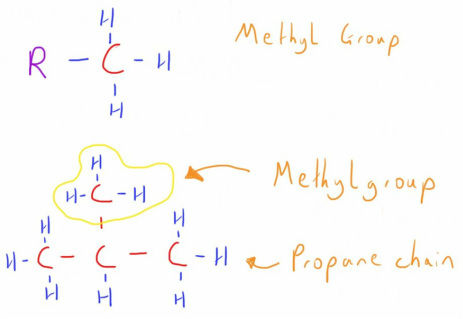
An Alkyl Group is simply an alkane molecule with one hydrogen atom removed.
As can be seen, this allows it to attached to other molecules through the exposed carbon atom.
They are named using the same formula as with alkanes, except the suffix is now '_yl'
e.g. Methyl group, Butyl group
As can be seen, this allows it to attached to other molecules through the exposed carbon atom.
They are named using the same formula as with alkanes, except the suffix is now '_yl'
e.g. Methyl group, Butyl group
Functional Groups
So now that we have an idea about the most basic organic molecules we can start to add different functional groups to add complexity.
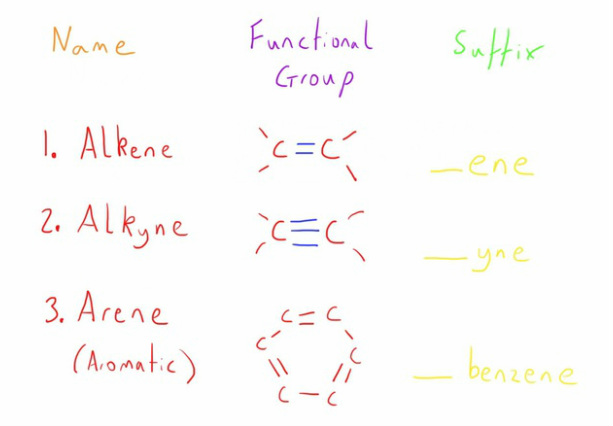
First lets look at the different shapes and bonds that the carbon atoms by themselves can take.
First lets look at the different shapes and bonds that the carbon atoms by themselves can take.
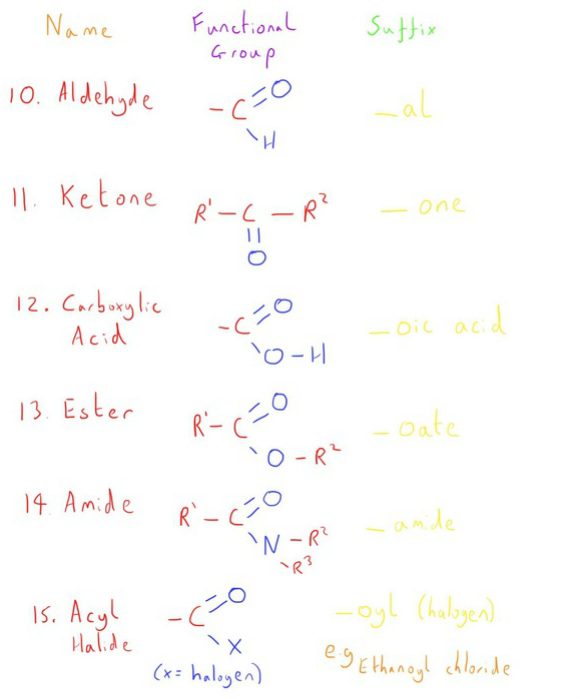
Now lets look at the functional groups that contain atoms other than carbon and hydrogen, specifically those of an electronegative nature.
Finally, let's look at the functional groups that arise from molecules with a carbonyl group.
A carbonyl group is where an oxygen atom is double bonded to a carbon atom.
A carbonyl group is where an oxygen atom is double bonded to a carbon atom.
Naming Rules
Well that's a bit to take in. Hopefully now though we have some tools to start making sense of some of the organic molecules that are commonly encountered.
Before we can run off and start naming all these molecules by ourselves we need to know a few rules about the ordering of the molecule names.
This is important in organic chemistry due to the large number of isomers possible.
Before we can run off and start naming all these molecules by ourselves we need to know a few rules about the ordering of the molecule names.
This is important in organic chemistry due to the large number of isomers possible.
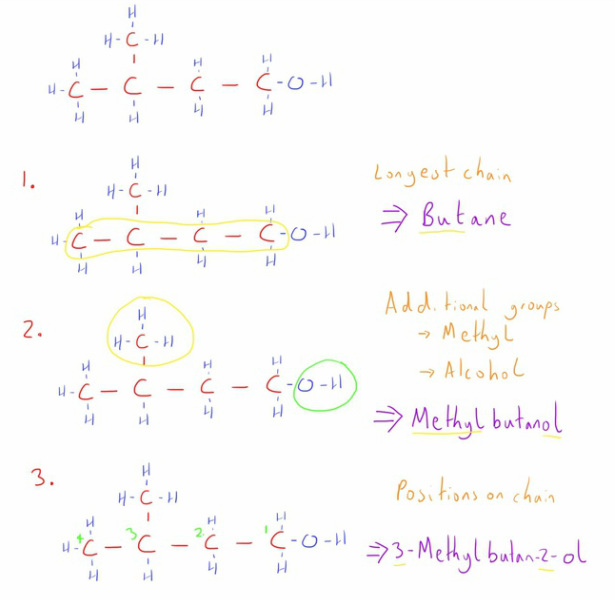
- Start with naming the longest carbon chain as an alkane.
- Identify an functional groups and alkyl groups outside the longest chain and add these as a prefix and/or suffix to the alkane you have.
- If there is more than position that the functional groups can occupy on the chain (i.e. it can form different structural isomers) then the groups' position on the main carbon chain is noted with a number. Numbering starts from the end of the chain that gives the lowest possible numbers for the groups.
- If there is more than one functional or alkyl group then they are listed in alphabetical order. e.g. 4-ethyl-2-methylhexane.
Hopefully that is more than enough to get us started and let us explore the different molecules in organic chemistry with a solid understanding of the basic language used.