Warfarin
Warfarin is a coumarin derivative that has it's mechanism of action by disrupting the synthesis of vitamin K dependant clotting factors.
These clotting factors are II, VII, IX and X.
Vitamin K is also required for synthesis of the anticoagulant factors Protein C and Protein S.
These clotting factors are II, VII, IX and X.
Vitamin K is also required for synthesis of the anticoagulant factors Protein C and Protein S.
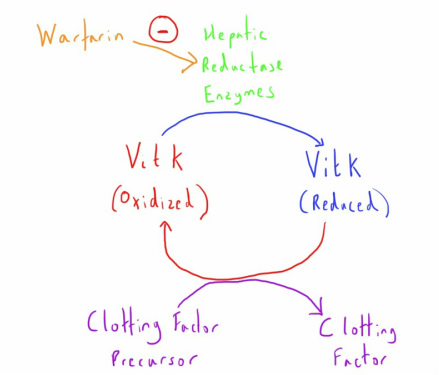
Vitamin K is necessary for the gamma-carboxylation of glutamic acid residues of the clotting factor precursor proteins in the liver.
To provide this function Vit K needs to be in the reduced state.
Warfarin inhibits the hepatic reductase enzyme that causes this transformation.
Without this reduced Vit K, synthesis of these clotting factors is impaired.
To provide this function Vit K needs to be in the reduced state.
Warfarin inhibits the hepatic reductase enzyme that causes this transformation.
Without this reduced Vit K, synthesis of these clotting factors is impaired.
The onset of warfarin's action will be delayed until the pre-existing factors are depleted.
Factor VII has the shortest half life of the clotting factors as so is the first to be depleted (hence why the INR measuring the extrinsic pathway is the most useful way of monitoring warfarin).
The other factors, especially prothrombin, will take longer to be depleted and hence the full anticoagulation effect is not noted until several days after initiation.
Factor VII has the shortest half life of the clotting factors as so is the first to be depleted (hence why the INR measuring the extrinsic pathway is the most useful way of monitoring warfarin).
The other factors, especially prothrombin, will take longer to be depleted and hence the full anticoagulation effect is not noted until several days after initiation.
Pharmacokinetics
Warfarin is almost completely absorbed via the oral route.
It is very highly protein bound (>95%) which makes it vulnerable to being displaced, and therefore having increased activity, by certain drugs e.g. NSAIDs.
It is metabolised by the cytochrome P450 system of the liver with conjugated products that undergo biliary and some renal excretion.
The half life of warfarin is long at 24-36 hours.
It is very highly protein bound (>95%) which makes it vulnerable to being displaced, and therefore having increased activity, by certain drugs e.g. NSAIDs.
It is metabolised by the cytochrome P450 system of the liver with conjugated products that undergo biliary and some renal excretion.
The half life of warfarin is long at 24-36 hours.
It is important to note that the mechanism of action is not related to the pharmacokinetics of warfarin itself but is dependant on warfarin's impact on the clotting factors.
Adverse Effects
- Haemorrhage - Anticoagulation is the obvious goal of warfarin therapy but this causes problems when there are drug interactions that affect it's action or unforeseen event e.g. trauma, emergency surgery.
- Drug Interaction - One of the most common problems as warfarin's clinical effects can be impacted by a large number of drugs and substances that interact with plasma protein binding, P450 metabolism, Vitamin K uptake or that impact on other components of haemostasis. Common culprits include NSAIDs (compete for protein binding), some antibiotics (induce or compete for P450 system) and antiplatelets (increased incidence of bleeding).
- Teratogenicity - Crosses the placenta and can cause developmental problems (most commonly in the first trimester) or perinatal bleeding (if used in the third trimester).
Clinical Application
The large variety in individual responses to warfarin require it's effects to be monitored.
This is done using INR measurements, with different targets for different clinical condition (a range of 2.0-3.0 is most common, with higher targets for conditions such as metal heart valves).
For similar reasons, careful initiation and titration is needed when starting warfarin to minimize the risk of bleeding complications and most organisations have their own regimes for achieving this.
Initiation much be especially careful in those with increased risk factors for supra-therapeutic levels e.g. chronic liver disease with pre-existing impairment of both clotting factors and plasma proteins for drug binding.
This is done using INR measurements, with different targets for different clinical condition (a range of 2.0-3.0 is most common, with higher targets for conditions such as metal heart valves).
For similar reasons, careful initiation and titration is needed when starting warfarin to minimize the risk of bleeding complications and most organisations have their own regimes for achieving this.
Initiation much be especially careful in those with increased risk factors for supra-therapeutic levels e.g. chronic liver disease with pre-existing impairment of both clotting factors and plasma proteins for drug binding.
Overdose
Management of overdose of warfarin, as measured by an excessively high INR, will depend on the level of the elevation and whether there is any resulting bleeding.
A patient with an elevated INR who is actively bleeding will be treated very differently from a patient who is asymptomatic with the same INR value.
In general there are 3 options:
A patient with an elevated INR who is actively bleeding will be treated very differently from a patient who is asymptomatic with the same INR value.
In general there are 3 options:
- Simply withhold or reduce subsequent warfarin doses and allow a gradual return to the target range. This is the best approach in mild elevations without bleeding.
- Vitamin K1 (Phytomenadione) can be given when reversal is required but isn't needed immediately. A small oral dose (0.5 -2.5mg) will allow utilisation of the Vitamin K by the liver for subsequent clotting factor synthesis and so reversal of warfarin's effect. This is useful for patients with a more worrying INR (e.g. higher than 8.0) but without active bleeding.
- Rapid reversal may be needed when there is active bleeding occurring in the presence of an abnormal INR. Whilst higher dose intravenous Vitamin K allows more rapid synthesis of clotting factors, there is still some degree of delay whilst these are synthesised (perhaps up to 6 hours). If this is too long then clotting factors need to be administered to the patient. This can be in the form of fresh frozen plasma, or more specific clotting factor formula (recombinent factor VII, Prothrombin complex concentrate).