The Gas Laws
There's no escaping it, some physiology requires the use of equations. This is particularly true for respiratory physiology where a good understanding of the behaviour of gases will help you understand the harder concepts. Here are a few of the most important gas equations for respiratory physiology, each with a brief description.
The Ideal Gas
The gas laws describe the behaviour of gases in different situations.
The all describe the actions of the so called ideal gas, with certain assumptions about its properties:
The all describe the actions of the so called ideal gas, with certain assumptions about its properties:
- There are no intermolecular forces between the individual molecules
- The molecules have no volume
- The molecule move constant, random motion
Fortunately, the behaviour of most gases approximates closely to this description of the ideal gases.
It is only really at very low temperatures and very high pressures that most gases diverge from these characteristics.
It is only really at very low temperatures and very high pressures that most gases diverge from these characteristics.
Kinetic Theory
The gas laws arise from the concepts of kinetic theory (check out the page on it if you haven't yet).
I find that keeping this idea in your mind helps provide an intuitive basis for the gas laws.
I find that keeping this idea in your mind helps provide an intuitive basis for the gas laws.
To summarise this concept for the gas laws:
From these principles it follows that:
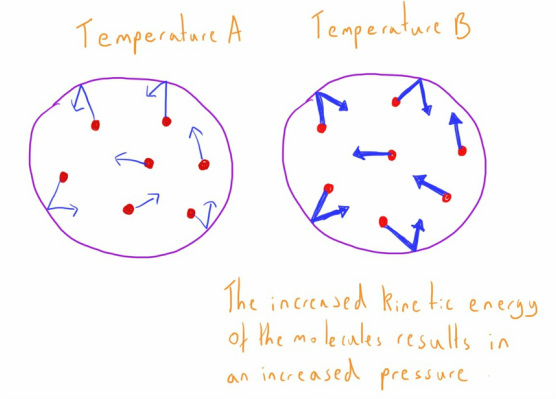
- Molecules are in constant motion (possess kinetic energy).
- The temperature of a substance is a measure of the average energy possessed by the molecules.
- When a molecule collides with the surface of its contain it will exert a force due to this kinetic energy.
- The force exerted by these multiple collisions across the surface of the container is the pressure exerted by the gas (pressure = force/area).
From these principles it follows that:
- An increase in temperature will provide more molecules with a higher kinetic energy.
- Molecules with higher energy will exert more force with the wall of the container when the collide with it, and therefore exert a higher pressure.
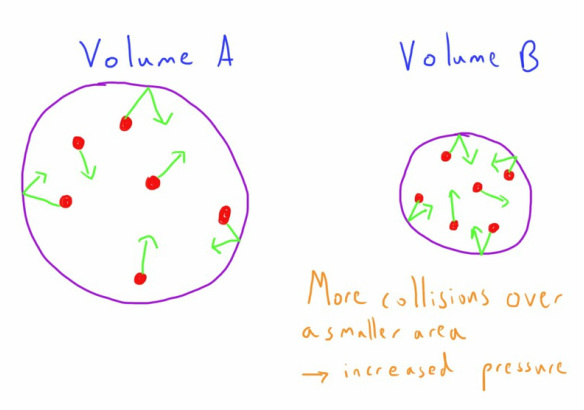
- If the volume of a container is smaller the molecules within will collide with the side more frequently.
- A smaller volume container will have a smaller surface area.
- A smaller volume therefore has a higher force transmitted over a smaller area and so result in a higher pressure.
The Three Gas Laws
The first gas laws (sometimes called the three gas laws), simply describe the above concepts of kinetic theory in a formulaic way.
The describe the relationships of the three important variables of a gas: pressure, volume and temperature.
The describe the relationships of the three important variables of a gas: pressure, volume and temperature.
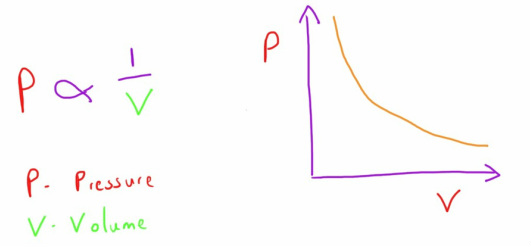
Boyle's Law
This law describes the relationship between the pressure and volume of a gas at a constant temperature.
It states that, at a constant temperature, the pressure and volume of a gas are inversely related.
i.e. if the volume of a gas is increased, the pressure of that gas will decrease.
if the volume of a gas is decreased, the pressure of that gas is increased.
It states that, at a constant temperature, the pressure and volume of a gas are inversely related.
i.e. if the volume of a gas is increased, the pressure of that gas will decrease.
if the volume of a gas is decreased, the pressure of that gas is increased.
If the volume is smaller then the molecules will collide with the wall of the container more frequently, transmitting more force and therefore exerting a higher pressure.
To help me remember the name of the law I was taught to remember that 'water Boyle's at a constant temperature'
To help me remember the name of the law I was taught to remember that 'water Boyle's at a constant temperature'
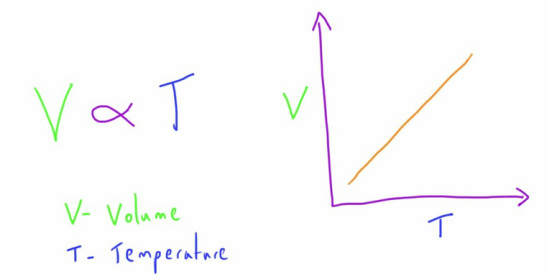
Charles' Law
This law follows on by describing the relationship between the temperature and volume of a gas at a constant pressure.
It states that, at a constant pressure, a change in temperature will result in a proportional change in volume.
i.e. if the temperature of a gas is increased, the volume of it will increase.
if the temperature of a gas is decreased, the volume of it will decrease.
It states that, at a constant pressure, a change in temperature will result in a proportional change in volume.
i.e. if the temperature of a gas is increased, the volume of it will increase.
if the temperature of a gas is decreased, the volume of it will decrease.
At a higher temperature the molecules have a higher average kinetic energy with increased movement that will move apart more.
This results in the gas occupying a higher volume.
To help me remember the name of the law I was taught to remember that 'Prince Charles is under constant pressure to become king'.
This results in the gas occupying a higher volume.
To help me remember the name of the law I was taught to remember that 'Prince Charles is under constant pressure to become king'.
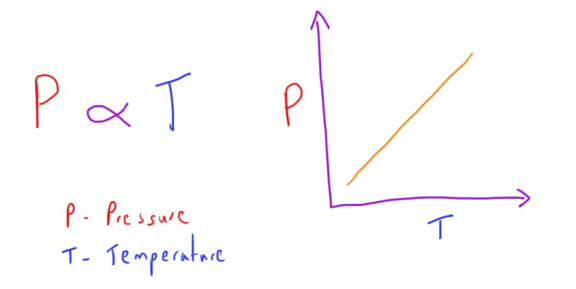
Gay-Lussac's Law
This law wraps up the three laws by describing the relationship between the temperature and pressure of a gas at a constant volume.
It states that, at a constant volume, a change in temperature will result in a proportional change in volume.
i.e. if the temperature of a gas is increased, the pressure of it will increase.
if the temperature of a gas is decreased, the pressure of it will decrease.
It states that, at a constant volume, a change in temperature will result in a proportional change in volume.
i.e. if the temperature of a gas is increased, the pressure of it will increase.
if the temperature of a gas is decreased, the pressure of it will decrease.
At a higher temperature the molecules have a higher average kinetic energy.
They collide with the container walls with greater force and therefore will exert a higher pressure.
They collide with the container walls with greater force and therefore will exert a higher pressure.
Avagadro's Law
To expand on the three gas laws we need to apply them to real gases, of which there are a large number, all very different.
Fortunately Avagadro noticed a very important principle that makes our life a lot easier.
Avagadro's Law basically states states that the volume that a gas occupies is dependant on the number of molecules and not the size/weight of those molecules.
The same number of molecules of different substances (but at the same temperature and pressure) will occupy the same volume.
This means that the same amounts of gas will behave the same as each other with regards to their volume, pressure and temperature.
All that will change is the mass of the gas, which will still be based on the molecular weight of the substance.
Avagadro's Law is based on the number of molecules of a substance.
For example, 32g of O2 will occupy the same volume as 44g of CO2 (as these are both 1 mole of the gas).
In case you're interested 1 mole of a gas will occupy approximately 22.4 Litres at STPD (Standard temperature, pressure and dry)
Fortunately Avagadro noticed a very important principle that makes our life a lot easier.
Avagadro's Law basically states states that the volume that a gas occupies is dependant on the number of molecules and not the size/weight of those molecules.
The same number of molecules of different substances (but at the same temperature and pressure) will occupy the same volume.
This means that the same amounts of gas will behave the same as each other with regards to their volume, pressure and temperature.
All that will change is the mass of the gas, which will still be based on the molecular weight of the substance.
Avagadro's Law is based on the number of molecules of a substance.
For example, 32g of O2 will occupy the same volume as 44g of CO2 (as these are both 1 mole of the gas).
In case you're interested 1 mole of a gas will occupy approximately 22.4 Litres at STPD (Standard temperature, pressure and dry)
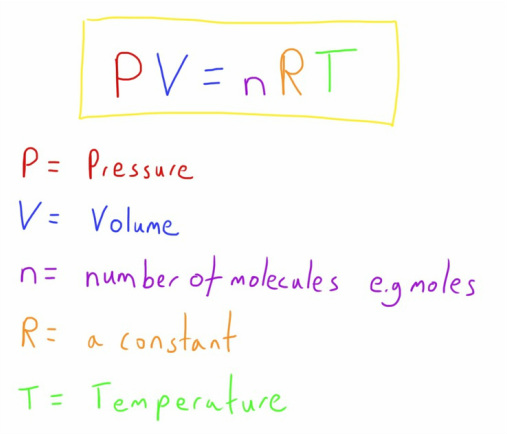
The Ideal Gas Law
With that final characteristic of gases explained we can put everything we know into one complete equation.
The equation highlights the interdependent relationship between pressure, volume, temperature and number of molecules in one nice equation.
A change in one value has to be compensated for by a change in another (or more than one other).
A change in one value has to be compensated for by a change in another (or more than one other).
Whilst that has covered the majority of the important concepts relating the the volume, pressure and temperature of gases, there are still a few more important laws to know about.
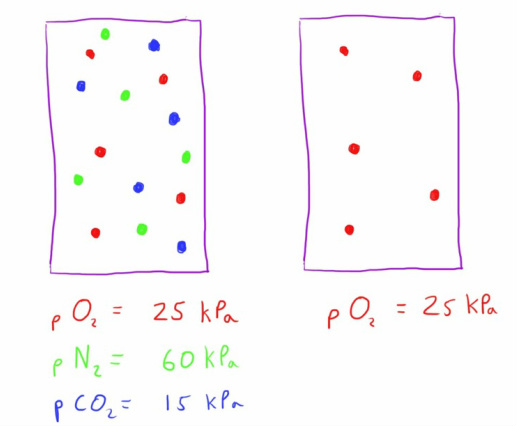
Dalton's Law
This describes the way in which a gas' concentration in a mixture of gases contributes to the total pressure of the gas.
Henry's Law states that the partial pressure exerted by a gas is the same as if the gas occupied the entire volume of the container by itself.
As a result, the molar fraction of the gas in the mixture can be used to calculate the partial pressure that the gas will exert.
Henry's Law states that the partial pressure exerted by a gas is the same as if the gas occupied the entire volume of the container by itself.
As a result, the molar fraction of the gas in the mixture can be used to calculate the partial pressure that the gas will exert.
From Avagadro's law we should remember that this is the molar fraction of the gas and the partial pressure is therefore determined by the number of molecules of the gas, regardless of their different sizes and weights.
Again, this is for ideal gases but is generally true is situations that we encounter.
Again, this is for ideal gases but is generally true is situations that we encounter.
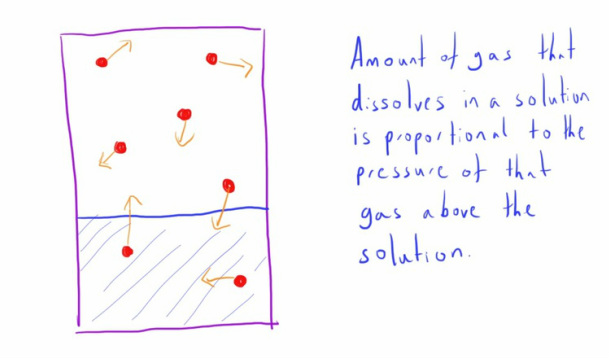
Henry's Law
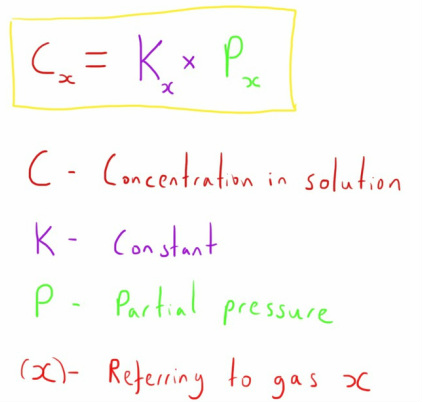
This states that 'The concentration of a gas that is dissolved in a solution is proportional to the partial pressure of that gas in equilibrium with the fluid (at a constant temperature)'.
It can be represented in equation form as below:
It can be represented in equation form as below:
This is important as it lets us know a bit about what the concentration of a gas in a liquid is going to be like, based on the partial pressure of a gas that is in contact with it (e.g. the concentration of CO2 in blood that is in contact with alveolar gas).
As you can appreciate this equation is starting to tell us a bit more about the solubility of gases.
Whilst this solubility is proportional to the partial pressure you can clearly see that the constant (K) will also have a big impact on the amount of a gas that ultimately ends up dissolved in the solution.
The value of K is dependant on several different factors including the physical properties of the gas, the solvent, and will be different at different temperatures (in general gases are more soluble at lower temperatures, the opposite of solids).
For instance, CO2 is much more soluble than O2 (about 20 times more), though its solubility will still be proportional to its partial partial pressure.
As you can appreciate this equation is starting to tell us a bit more about the solubility of gases.
Whilst this solubility is proportional to the partial pressure you can clearly see that the constant (K) will also have a big impact on the amount of a gas that ultimately ends up dissolved in the solution.
The value of K is dependant on several different factors including the physical properties of the gas, the solvent, and will be different at different temperatures (in general gases are more soluble at lower temperatures, the opposite of solids).
For instance, CO2 is much more soluble than O2 (about 20 times more), though its solubility will still be proportional to its partial partial pressure.