Kinetic Theory
There are some fairly basic concepts covered in this chapter but I find that a good understanding of these concepts really helps with many of the other physics topics that we shall cover, especially the gas laws.
All molecules (except those at the temperature absolute zero) have a degree of kinetic energy.
They are in constant movement.
The temperature of a substance is simply a measure of the average kinetic energy of the molecules.
They are in constant movement.
The temperature of a substance is simply a measure of the average kinetic energy of the molecules.
The more kinetic energy the molecules have (and therefore the higher the temperature of the substance), the more they will vibrate and move around.
This scale of kinetic energy produces the different states (also called phases) of matter.
This scale of kinetic energy produces the different states (also called phases) of matter.
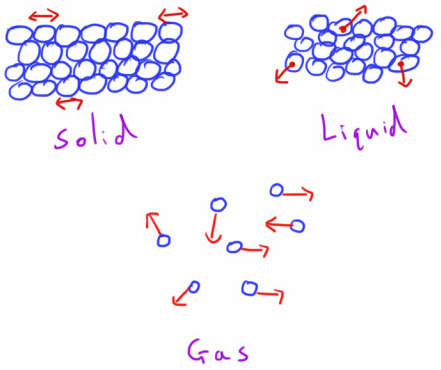
States of Matter
In a Solid, the molecules have a lower amount of kinetic energy. They still vibrate, but the intermolecular forces are strong enough to result in a rigid, organised structure where the molecules are tightly packed together.
In comparison, a Liquid of the same substance had a higher temperature, and the molecules have (on average) greater kinetic energy.
This kinetic energy is enough to overcome the intermolecular forces to some degree and allow free-er movement with molecules moving around past each other but still being kept relatively close to each other.
This kinetic energy is enough to overcome the intermolecular forces to some degree and allow free-er movement with molecules moving around past each other but still being kept relatively close to each other.
The Gas phase of a substance has an even higher temperature and the molecules even greater average kinetic energy.
This kinetic energy is great enough to (almost) completely overcome the intermolecular forces and the molecules move around in free, random motion, colliding with each other and the walls of the container.
This kinetic energy is great enough to (almost) completely overcome the intermolecular forces and the molecules move around in free, random motion, colliding with each other and the walls of the container.
Pressure and State of Matter
The above descriptions of states of matter describe the impact of temperature of a substance on the matters states.
Whilst this is the most important determinant, the pressure the substance is under is also important, mostly when it comes to the gaseous state.
This can be clearly understood by seeing how the increased pressure is able to oppose the increased kinetic energy and movement of the molecules.
Whilst this is the most important determinant, the pressure the substance is under is also important, mostly when it comes to the gaseous state.
This can be clearly understood by seeing how the increased pressure is able to oppose the increased kinetic energy and movement of the molecules.
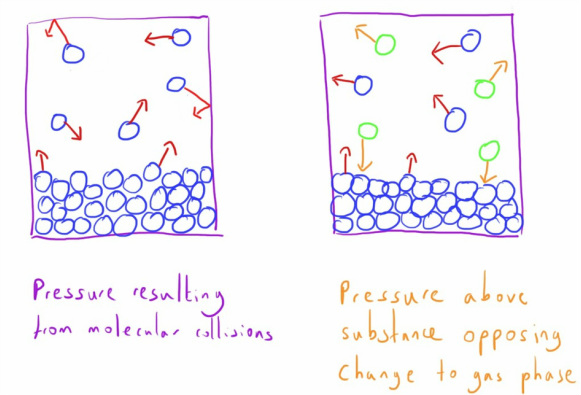
Indeed it is the kinetic energy of the molecules that is responsible for the pressure that gases exert.
Pressure is defined as force exerted per area (see basics of measurement)
The force is from the kinetic energy of the molecules when they collide with the sides of the container.
This is talked about in more detail when we cover the gas laws.
Pressure is defined as force exerted per area (see basics of measurement)
The force is from the kinetic energy of the molecules when they collide with the sides of the container.
This is talked about in more detail when we cover the gas laws.
As such, at an increased pressure a higher temperature is needed to change a substance from the liquid state to the gaseous one.
The same is true for the solid to liquid state, though the impact is less dramatic given the reduced compressibility of liquids.
The same is true for the solid to liquid state, though the impact is less dramatic given the reduced compressibility of liquids.
A good example of this is the example of boiling water at higher altitudes.
At the summit of Everest, for example, the atmospheric pressure is significantly lower than at sea level (where water boils at 100 degrees Celsius - in case you weren't sure).
As a result, water boils at 71 degrees Celsius instead.
At the summit of Everest, for example, the atmospheric pressure is significantly lower than at sea level (where water boils at 100 degrees Celsius - in case you weren't sure).
As a result, water boils at 71 degrees Celsius instead.
Vapours and Gases
There is an area of potential confusion when the terminology of vapours and gases starts to be used.
This arises from the interaction between pressure, temperature and the states of matter.
This arises from the interaction between pressure, temperature and the states of matter.
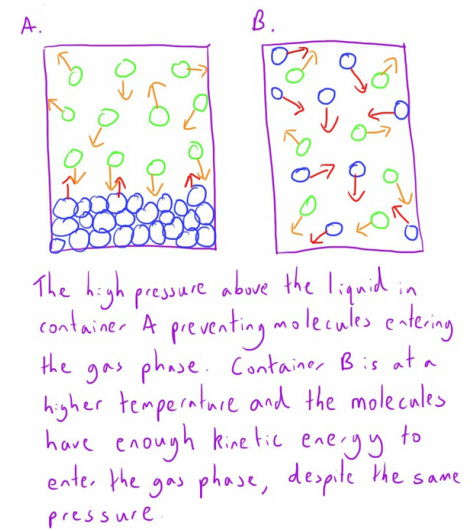
The Critical Temperature of a substance is the temperature at which a substance will exist in the gaseous phase regardless of the amount of pressure applied.
At a molecular level this can be seen as the point when the molecules have so much kinetic energy that no amount of pressure can force the molecules to maintain a fluid state.
At a molecular level this can be seen as the point when the molecules have so much kinetic energy that no amount of pressure can force the molecules to maintain a fluid state.
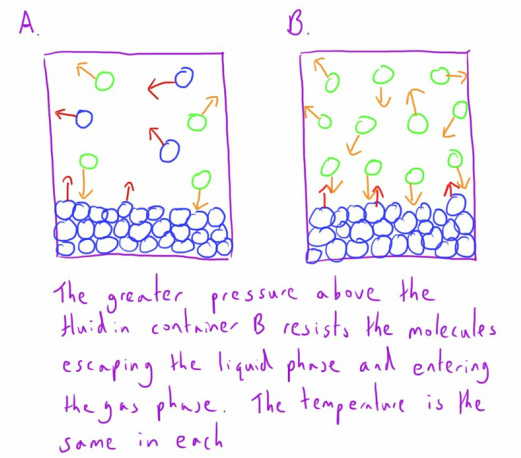
In the diagram above, if the substance was below it's critical temperature, then if the pressure it was under was increased sufficiently it would be forced into the liquid phase, as in container A.
If the substance was above its critical temperature then regardless of the pressure it was subjected to it would exist in the gaseous phase as the molecules have so much kinetic energy.
If the substance was above its critical temperature then regardless of the pressure it was subjected to it would exist in the gaseous phase as the molecules have so much kinetic energy.
Why is this relevant? Well a gas is a substance that is above it's critical temperature and so will always exist in the 'gas state' regardless of how much pressure is applied to it.
Compare this to a vapour which is a substance in the gaseous state that is below it's critical temperature.
This means that whether it is in the liquid or the gaseous state will depend on the pressure it is under.
Compare this to a vapour which is a substance in the gaseous state that is below it's critical temperature.
This means that whether it is in the liquid or the gaseous state will depend on the pressure it is under.
Saturated Vapour Pressure
Whilst the description so far makes it sound as if a substance is in either one state of matter or another, this is not quite correct.
As mentioned, temperature is a measure of the average kinetic energy of the molecules of a substance.
This implies that some molecules have a greater kinetic energy than others, and thus some of these will have enough to escape this liquid phase and exist in the gaseous phase.
As mentioned, temperature is a measure of the average kinetic energy of the molecules of a substance.
This implies that some molecules have a greater kinetic energy than others, and thus some of these will have enough to escape this liquid phase and exist in the gaseous phase.
An equilibrium will therefore exist between the liquid and gas phase (and to a lesser degree the solid and liquid phases and the solid and gaseous phases).
The position of this equilibrium (how much of the substance is in each phase) will depend on the temperature which the substance is at.
This position is measured as the Saturated Vapour Pressure, and will be dependant on the temperature of the substance.
This is because the molecules in the gaseous phase will exert a pressure that is based on their kinetic energy.
The position of this equilibrium (how much of the substance is in each phase) will depend on the temperature which the substance is at.
This position is measured as the Saturated Vapour Pressure, and will be dependant on the temperature of the substance.
This is because the molecules in the gaseous phase will exert a pressure that is based on their kinetic energy.
It is the point when the saturated pressure is equal to the atmospheric pressure that a substance will boil, as the molecules are exerting enough pressure to oppose the atmospheric pressure.